What is Road Salt?
North America started using road salt in the 1940s and since then chloride concentrations in ground water, even in the summer months, have greatly increased. Road salt is a sodium chloride product which can contain magnesium chloride, calcium chloride, and/or potassium chloride. Road salt provides traction for our feet and tires by changing the melting point of the ice so that it melts in freezing temperatures. North America started using road salt in the 1940s and since then chloride concentrations in ground water, even in the summer months, have greatly increased [1]. The water quality standard in Illinois for chloride concentrations states that the concentrations should not be greater than 500 mg/l in freshwater environments (Title 35, Subtitle C, Chapter I, Section 302.208 of the Illinois code). However, throughout the years, in many urban and suburban areas, the concentration of chloride has been found to be much higher.
How does Road Salt Impact Our Waterways?
Although road salt can keep us from slipping, we must ask ourselves what does road salt do to our aquatic ecosystem? It might not come as a surprise that road salt isn’t the best thing for aquatic ecosystems or our water quality. This is because the salt dissolves as part of the ice melting which means that the sodium chloride, magnesium chloride, calcium chloride, and/or potassium chloride ends up getting into our soil and thus to ground water sources or gets washed into our storm drains which lead to our local waterways.
Freshwater animals are adapted to having low salt levels in their habitats. Thus, when salt concentration increases these animals have difficulty keeping water in their cells. This is because of osmosis which is the biological phenomenon where water moves through a semi-permeable membrane from a solution with a high concentration of water molecules to a solution with lower concentration of water molecules. This lack of water in aquatic animal’s cells cause the cells to shrivel up and die. The death of these cells cause injury and sometimes death to these aquatic animals[1]. Road salt isn’t just bad for aquatic animals though. It is bad for our pets as well. This is because, although our pets don’t like to eat the salt off the road, they get the salt on their feet which they then lick causing them to ingest the salt. Road salt is also highly corrosive which means it can cause damage to vehicles and infrastructure.
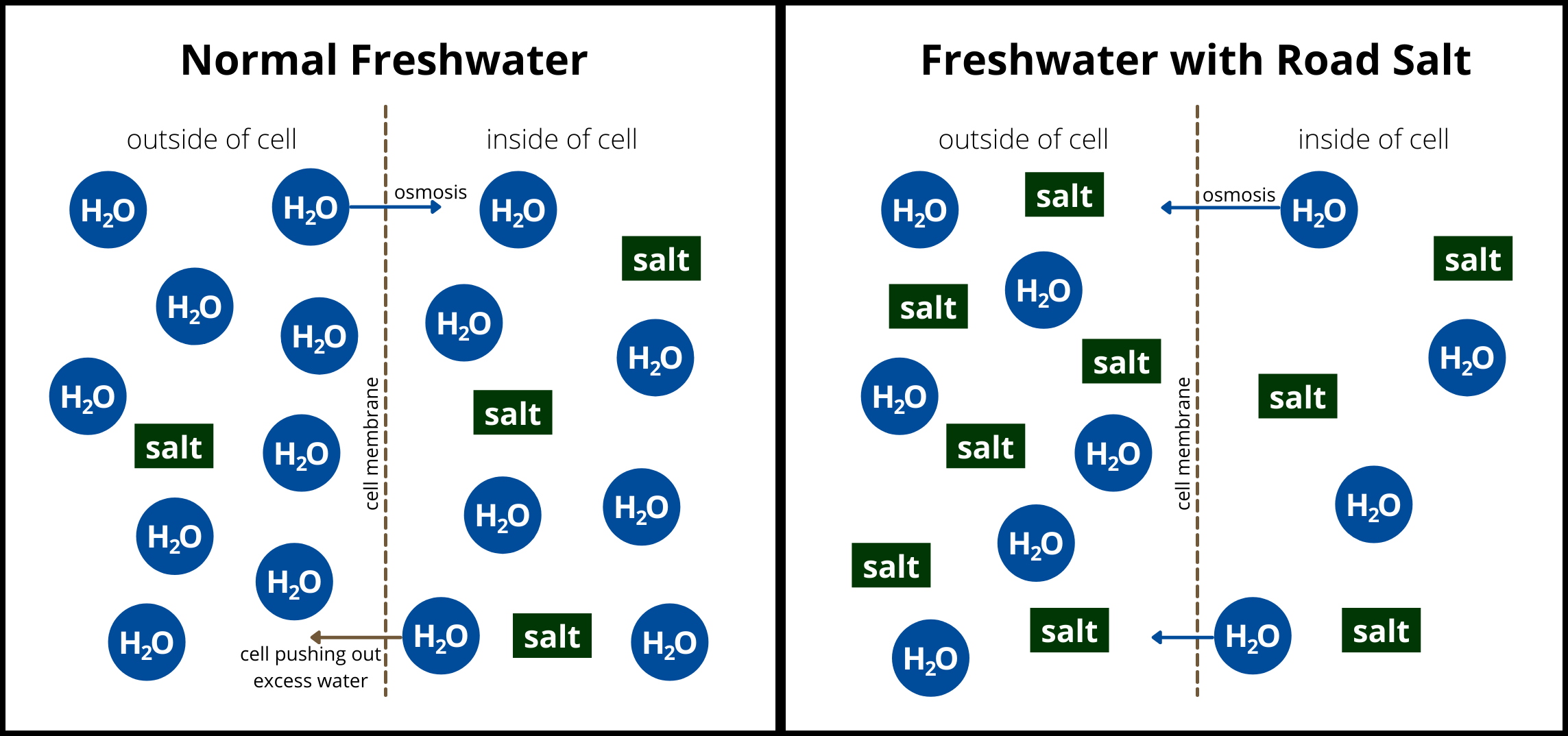
Figure 1: Adding Salt to Freshwater Changes How Water (H2O) Moves in and Out of Animal Cells. (2021). Illinois Riverwatch Network Chloride Monitoring Manual. Retrieved January 5, 2023, from http://www.ngrrec.org/Riverwatch/.
What Can We Do?
Since road salt isn’t great for our aquatic ecosystems, pets, infrastructure, and cars you might be wondering what you should use instead. Switching to a minimally toxic product like a urea based product or a carbonite or carbonyl based product is a great idea. They are easy to find at most local stores and online. If you are mostly worried about traction, try switching to sand, but make sure to spread in moderation as sand still gets into our waterways and is known to clog storm drains when in excess. Traditional ice picks and shoveling are also great ways to get rid of snow and ice with no fossil fuel usage or downstream impacts!
If you are still wanting to use salt, make sure to use it sparingly. The average driveway only needs about a 12 ounce coffee mug of salt. In temperatures below 15°F, rock salt does not work. Instead, switch to a deicer formulated for colder temperatures [2].

Figure 2: 4 Steps To Be Salt Smart. (2021). Salt Smart Collaborative. Retrieved December 15, 2023, from https://saltsmart.org/residents/
You can also make brine at home instead of using road salt. Using brine helps you to put less salt in our environment (Brine is actually only 23% salt) and can be added before it even snows [2]! All you need to make brine is road salt, a measuring cup, hot water, and something to spread the brine with like a pump sprayer.
First, heat up about one to two gallons of water. Then measure out 3 and a half cups of salt in a bucket and pour the hot water over the salt. Once water is covering the salt mix vigorously until all of the salt is dissolved. Let the water cool and pour brine into your sprayer using a strainer if you notice salt clumps in the bottom of the bucket. Then you are ready to use your brine 24-48 hours before it snows as long as rain is not forecasted before snow. To apply your brine spray lines back and forth across the pavement area. Just like with road salt, you do not need to cover the entire pavement. If you notice that there is salt setting in your sprayer shake it. Come spring, any leftover brine can be stored in a milk jug for next winter [2].
[1] Illinois RiverWatch Chloride Monitoring Manual. The National Great Rivers Research & Education Center Illinois RiverWatch Network. (2021, October). Retrieved January 5, 2023, from http://www.ngrrec.org/Riverwatch/ [2] Resources for Residents. Salt Smart Collaborative. Retrieved December 15, 2023, from https://saltsmart.org/residents/



